Team Leader:
What is a natural history study?
A natural history study (NHS) is a medical research study that collects comprehensive information about the development, symptoms, and lived experiences of people with a rare genetic condition. They are considered longitudinal observational studies, because they document the course of a genetic disorder in people over several years, during which time they receive standard treatments for these disorders. A typical NHS involves patients of all ages and may include people with varying degrees of disorder severity. Enrolling as many people into the study as possible helps scientists understand the full scope of a genetic condition. Importantly, these studies—whose goal is to improve prognosis and the quality of life for the patients as well as their families—are a means for the families of people with STXBP1 and SYNGAP1 disorders to actively participate in research, share their experiences, and contribute to the scientific understanding of these disorders. Dr. Ingo Helbig of CHOP leads the NHS for both STXBP1 and SYNGAP1.
Why are natural history studies important for STXBP1 and SYNGAP1 disorders?
Natural history studies are vital for rare disorders because they fill critical knowledge gaps, facilitate the development of treatments, improve patient care, empower patient communities, and contribute to advancements in healthcare policy.
Natural history studies for STXBP1 and SYNGAP1 disorders will help clinicians and researchers establish a baseline of information regarding clinical features, symptoms, and the developmental progression experienced by affected people. Data from these natural history studies will help to generate more effective care and management strategies for STXBP1 and SYNGAP1 patients, guide the development of targeted therapies, pinpoint appropriate timing for interventions, and determine relevant outcome measures for clinical trials. Indeed, clinical trials for rare disorders are constructed differently from trials for more common diseases because of the low incidence of rare disorders. NHS data improves our understanding of a genetic condition and therefore can inform which clinical endpoints or outcomes will be the most informative when designing novel clinical trials, which will ultimately determine whether a treatment or therapy is effective or not.
Collaboration among researchers, healthcare providers, patient advocacy groups, and pharmaceutical companies is often necessary for conducting NHSs on rare disorders. Such collaborations can pool resources, expertise, and patient populations to conduct meaningful research. These relationships are equally important to building (the foundations for?) clinical trial readiness in the STXBP1 and SYNGAP1 disorders community. Regulatory agencies, such as the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA), normally require natural history data before approving clinical trials to assess potential therapeutics. During clinical trials, NHS data is crucial for demonstrating the safety and efficacy of a given treatment.
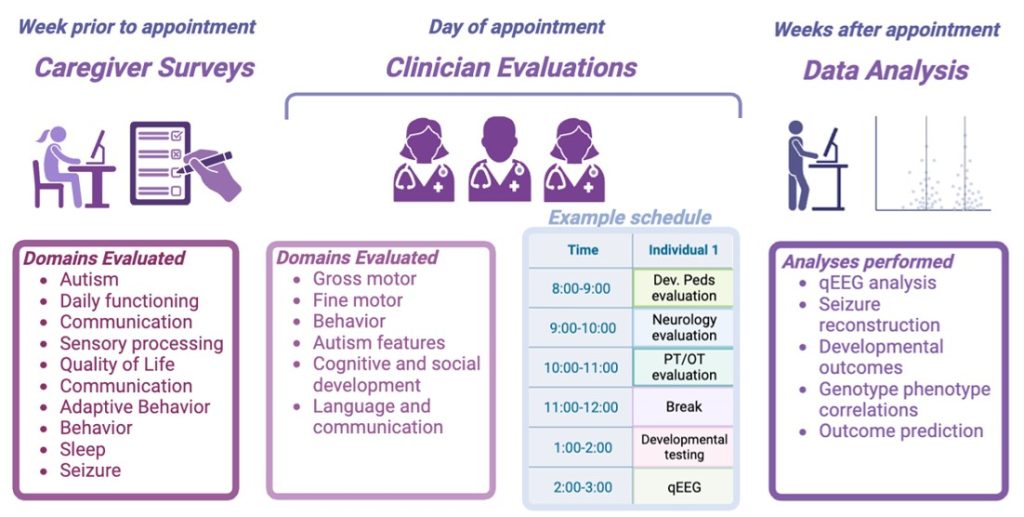
What does participation in the natural history study for STXBP1 and SYNGAP1 disorders look like?
When you and your child participate in the NHS for STXBP1 and SYNGAP1 disorders, you will have a day filled with both clinical and research appointments with dedicated teams at various study sites.
For SYNGAP1 disorders, there is currently one site: CHOP’s Epilepsy and Neurodevelopmental Disorders program (ENDD).
For STXBP1 disorders, there are currently four sites nationwide: CHOP’s Epilepsy and Neurodevelopmental Disorders program (ENDD), Children’s Hospital Colorado, Texas Children’s Hospital, and Weill Cornell Medicine. The STXBP1 disorders NHS is also known as the STARR study .
During your visit, you’ll meet with various healthcare professionals who specialize in caring for children with STXBP1 and SYNGAP1 disorders. These experts may include:
- Neurologist: Discussing topics like epilepsy management and medication.
- Genetic Counselor: Exploring genetic variant interpretations.
- Physical Therapist and Occupational Therapist: Offering guidance on developmental milestones and providing recommendations or plans for at-home therapists.
- Developmental and Behavioral Pediatrician or Neuropsychologist: Providing strategies for behavior management.
To ensure we address all of your concerns and provide tailored medical recommendations, please plan for a full day of appointments.
We are also interested in hearing from you and other caregivers who are closely involved in your child’s care. We’re partnering with a specialized company to collect caregiver-reported outcomes. These standardized scales provide insights into the day-to-day life of caring for a family member with these disorders, offering a unique perspective beyond measures typically collected in clinical appointments.
If you would like to enroll in either of our natural history studies, or would like more information, please contact us at endd@chop.edu.